Page Contents
WHAT IS IT?
Hemophilia B is an X-linked recessive genetic condition that is marked by a deficiency in clotting factor IX of the coagulation cascade. Sequence variants of the factor IX are the cause of most all cases.
WHY IS IT A PROBLEM?
Factor IX is an important clotting factor for the coagulation cascade. Its absence/deficiency predisposes the patient to bleeding that can be quite severe.
WHAT MAKES US SUSPECT IT?
Risk factors: male, family history
Common chief concerns: intuitively, many of the chief concerns patients with this condition will express revolve around episodes of bleeding.
Hematoma (head): these bouts of bleeding are common in young children who frequently experience bumps to the head.
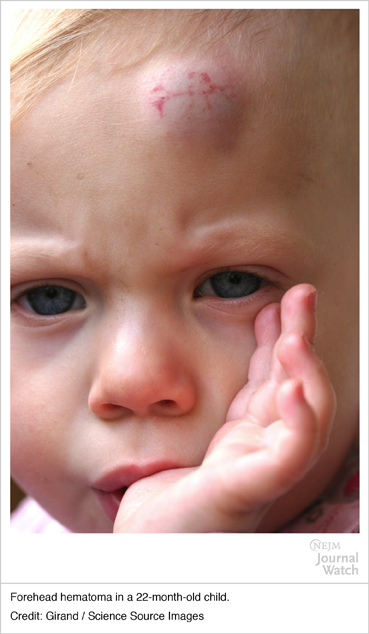
Easy/excessive bruising: such an observation should raise clinical suspicions for hemophilia
Prolonged bleeding after surgery: such an observation should raise suspicions for hemophilia (bleeding excessively after dental procedures is common)
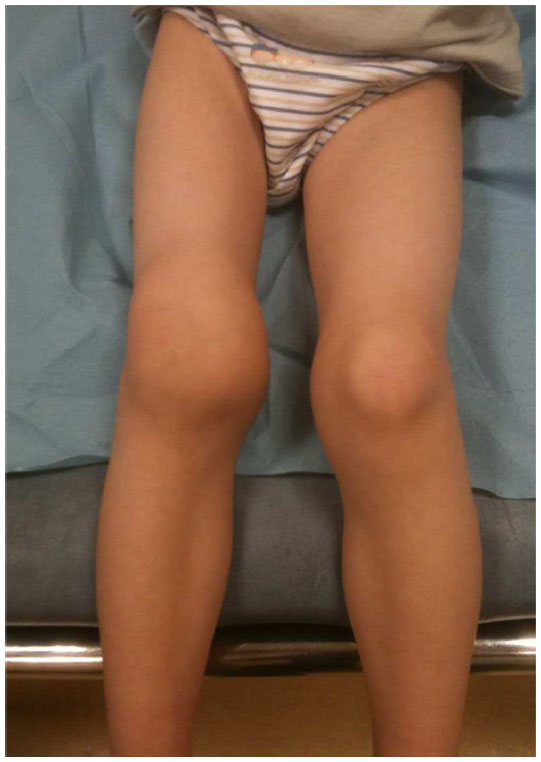
Hemarthroses: bleeding can occur in the joints (such as the knee) which can appear swollen on exam, and also can cause pain (and associated limping). Also called chronic hemophilic arthropathy, proteases form the blood in the synovial space will destroy joint cartilage.
HOW DO WE CONFIRM A DIAGNOSIS?
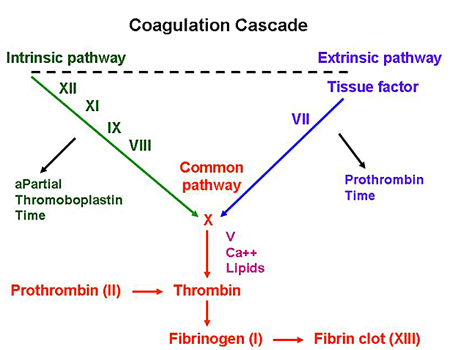
Coagulation studies: use this guide as a reference to interpret the below findings that one would see in hemophilia B.
- Increased PTT (factor IX is in the intrinsic pathway)
- Normal PT
- Normal BT
Factor IX activity assay: if the results of the coagulation factor are what is seen above, directly testing for factor IX activity can assess the likelihood of hemophilia B. Factor activity will be decreased in patients with this condition (and lower activities are associated with more severe disease). KEEP IN MIND THIS IS HOW WE CAN DISTINGUISH BETWEEN HEMOPHILIA A VS. B (they are initially clinically indistinguishable).
Genetic testing can be used to confirm the specific causal mutation for this condition.
HOW DO WE TREAT IT?
Factor IX concentrate: recombinant factor IX protein is given to patients with hemophilia B given that this is the clotting factor that they are missing.
HOW WELL DO THE PATIENTS DO?
This condition can be very well managed pharmacologically, and mortality of patients in resource rich parts of the world is comparable to that of the general population (source)
WAS THERE A WAY TO PREVENT IT?
While there is not way to prevent this genetic condition, screening potential female carriers of the disease can help inform patients on the risk of having children with hemophilia B.
WHAT ELSE ARE WE WORRIED ABOUT?
Intracranial hemorrhage while rare, this is the leading cause of bleeding-related death in patients with hemophilia B (source)
Patients can develop inhibitors (IgG antibodies that neutralize the clotting factor). Keep this in mind as it changes the treatment landscape for the patient! If a patient has inhibitors replacing the clotting factor will not be as beneficial. If a patient has inhibitors different therapy is needed (such as clotting factor VIIa).
OTHER HY FACTS?
If patients do not improve after recombinant factor IX supplementation, test them for the presence of factor IX inhibitors (antibodies that antagonize this factor which can develop over time in patients).
Normal hemophilia activity is between 50%-150%
Factor IX is vitamin K dependent
Page Updated: 01.09.2016