Page Contents
WHAT ARE THEY?
Broadly speaking, hematological malignancies refer to any type of cancer that originates from the blood cell lineage. There are many different types of hematological malignancies, and the classifications can become difficult to remember, however there is a very logical approach one can take clinically to diagnose these malignancies without too much trouble.
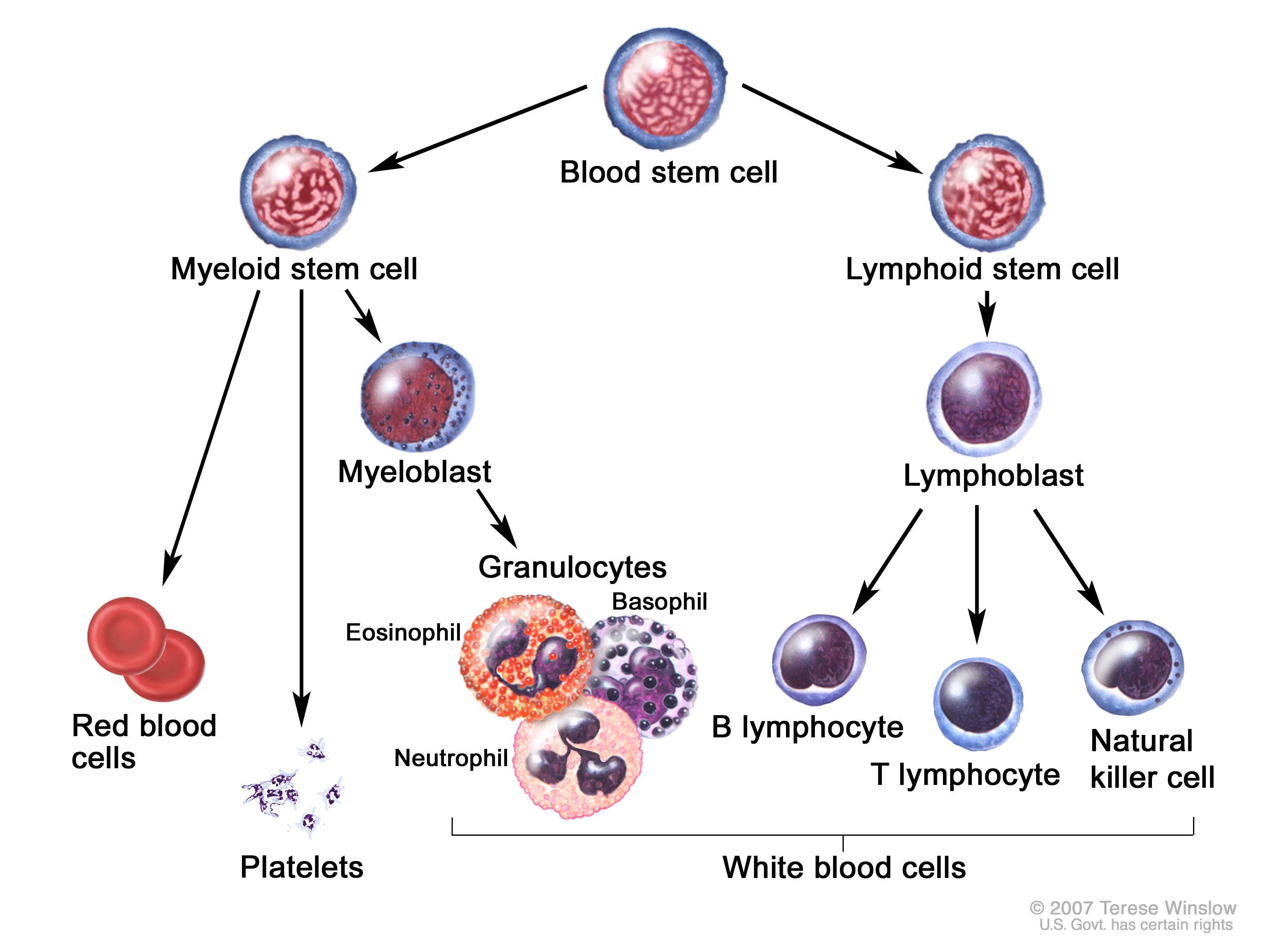
Before working through a diagnostic path, let us first introduce the different types of malignancies we will be discussing. Let us keep in mind that these neoplastic cells are clonal and do not mark reactive conditions:
- Leukemias: this category of cancers refers to malignancies asserted with early white blood cell (WBC) progenitors that originate and accumulate in the bone marrow and then spill into the circulating blood, while also possible invading other tissues. Leukemias can be of either myeloid or lymphoid origin, and can also present acutely or chronically (CML, CLL, AML, ALL).
- Lymphomas: these are similar to leukemias, however they typically originate in the lymph nodes and usually do not release cells into the blood. The majority of lymphomas are of B-cell origin, however they can be caused by T-cells as well. This is a diverse group of neoplastic disorders (Hodgkin lymphoma, diffuse large B-cell lymphoma, and Burkitt Lymphoma are a few examples).
- Chronic myeloproliferative disorders: these are a more specific type of hematological malignancy that deal exclusively with the myeloid lineage, and chronic diseases. These neoplasms (like leukemias) originate in the bone marrow and can eventually release cells into the blood. The difference is that the myeloid lineage produces more than just white blood cells, and issues with erythroid and platelet precursors also fall under this category of disease (such as myelofibrosis). *Because chronic myeloid leukemia (CML) fits this criteria, it is classified both as a leukemia and a chronic myeloproliferative disorder.
- Plasma cell neoplasms: another fairly specific category of hematological malignancy, plasma cell neoplasms are composed (at least partially) of terminally differentiated B-cells (i.e. plasma cells). These cancers also originate in the bone marrow (multiple myeloma).
- Myelodysplastic syndromes: this category is very heterogenous and poorly understood. These are also found in the bone marrow, and result from abnormal (dysplastic) maturation of myeloid bone marrow progenitors.
WHY ARE THEY A PROBLEM?
Depending on where the neoplasm is, excessive growth of a hematopoietic cell lineage can cause many different issues for the patient (and disrupt normal tissue function). A common example of this is that the growth of neoplastic cells within the bone marrow will impair normal hematopoiesis. This can lead to a deficiency of mature WBCs, red blood cells (RBCs), and platelets predisposing the patient to infection, anemia, and bleeding.
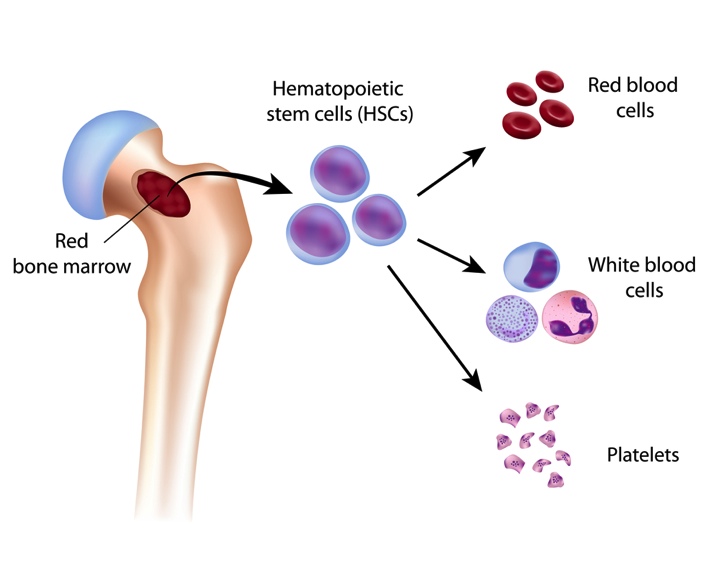
WHAT MAKES US SUSPECT THEM?
While hematological malignancies within themselves have a very broad clinical presentation (given so many conditions that fall under this category of disease!) there are some clinical presentations that should cause a physician to consider the possibility of a blood cell related cancer.
Chief complaints: Constitutional symptoms (unexplained by other processes) including weight loss, fevers, fatigue, night sweats, and malaise.
Physical Exam:
- Lymphadenopathy (swollen lymph nodes that are often painless) that is persistent.
- Splenomegaly that is unexplained by another condition.
*Often these malignancies can be asymptomatic/detected randomly by some of the general tests ordered: if a patient is referred to you with these general findings, keep the possibility of cancer in the back of your mind.
- Abnormal blood counts (WBCs, RBCs, platelets)
- Presence of unusual cells on peripheral blood smear
WHAT INITIAL CLINCIAL TESTS SHOULD ONE ORDER?
Both a complete blood count (CBC) as well as a peripheral blood smear are important initial tests to order (and may already have been performed on the patient for some other reason).
CBC: this exam will give you much information about the status of all the blood cells in the patient. Given that each specific hematological malignancy presents differently on the CBC, here are some general findings to guide you.
- Cytopenias including neutropenia, thrombocytopenia, anemia, and pancytopenia can be indicative of issues with the bone marrow leading to complications with hematopoiesis
- Thrombocytosis (sometiems in combination with cytopenias) can be seen in CML however this is usually not seen in many other malignancies.
- Leukocytosis is possible especially with acute leukemias
- Polycythemia: the presence of increased RBCs can be suggestive of polycythemia vera
Blood smear: this is an easy test to perform and can be helpful in a diagnostic path. It is important to note that the absence of abnormalities does not rule out malignancy (neoplastic cells may not be in the blood). Below are some findings that can aid in the diagnosis.
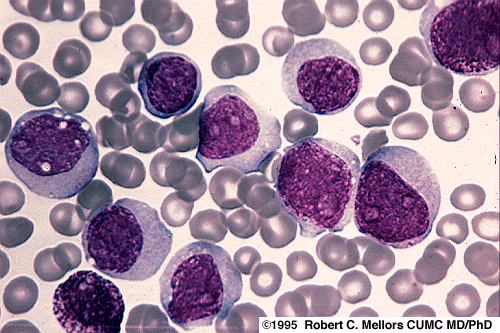
Increased myeloblasts can be suggestive of a lymphoma or a leukemia such as AML.
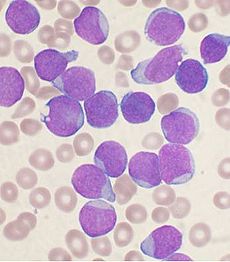
Increased lymphoblasts: can be suggestive of a lymphoma or a leukemia such as ALL.
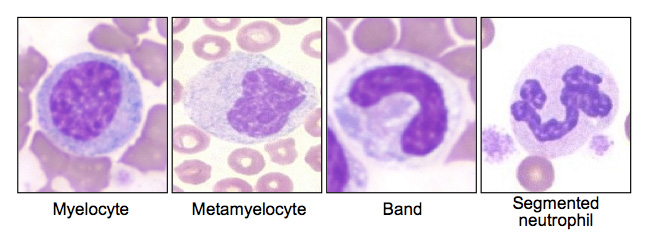
“Left Shift” on a blood smear refers to the presence of immature WBCs (specifically neutrophils) on the smear. This is suggestive of either an increased demand for these cells, or a disease in the bone marrow that is physically “pushing out” WBCs before they have time to fully mature (leukemia).
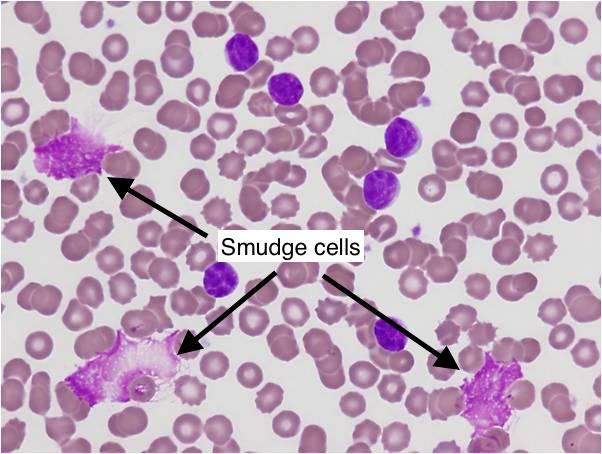
“Smudge Cells”: These are fragile lymphocytes (which are clonal B-cells produced in CLL) that are small, round , and have small cytoplasms. They are physically crushed during the process of preparing the blood smear slide (resulting in their characteristic appearance).
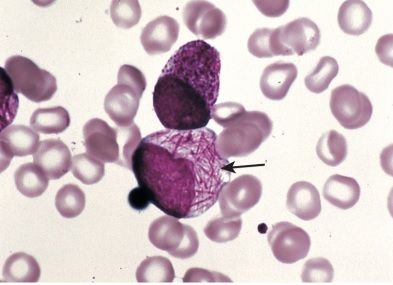
Auer rods: these are cells found in acute promyelocytic leukemia (a form of AML) that are very specific to this disease.
WHEN DO WE BIOPSY?
If our initial tests (CBC and/or blood smears) arouse our clinical suspicion about the possibility of the patient having a hematological malignancy (or if we know one exists and want to further characterize it) often a biopsy will be required. We must get tissue for further histological analysis in order to confirm the diagnosis of many hematological malignancies. The source tissue can either be the bone marrow, lymph node, or even an external mass. The decision behind which tissue to biopsy isn’t the most difficult, however here are some general guidelines:
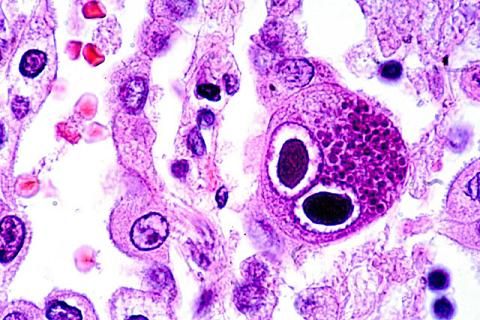
- Lymph nodes biopsies: If the physical exam, or imaging reveals any suspicious lymph nodes, it is likely that the tissue should be biopsied (the CBC and blood smear can help inform this decision). Specific histological findings can provide us with a likely diagnosis (such as Reed-Sternberg cells which are specific to Hodgkin lymphoma).
- Bone marrow biopsies should be conducted when the CBC/blood smear is suggestive of a neoplasm that has found its way into the bone (either displacing normal cells, releasing malignant cells into the blood stream, and/or causing primary bone marrow failure). Histological analysis can demonstrate the causal malignant cell, and also can reveal specific pathological features that can aid in a diagnosis (such as fibrosis in myelofibrosis)
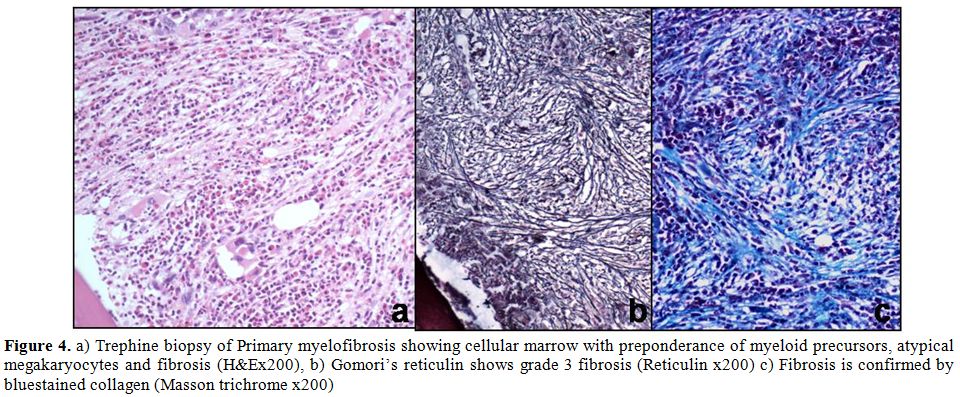
- External masses (extranodular): various hematological malignancies can cause solid tumors to form outside of the bone marrow/lymph nodes. If a suspicious mass is found on physical exam/imaging a biopsy of the tissue might be beneficial.
WHAT OTHER TESTS SHOULD BE CONDUCTED?
Cell surface marker analysis: After (or alongside some of the above studies) it can be very advantageous to perform flow cytometry/immunohistochemsitry in order to analyze the different cell surface marker expression of malignant cells (either from whole blood, tissue biopsy, or bone marrow aspirate). While all of the exact cell marker expression patterns are too detailed to explain here, the most important thing to realize is that clinically we do not only have to rely upon morphology in making our diagnosis (we can use the presence or absence of cell surface markers to inform what lineage our malignant cells belong to).
Example: in diffuse large B-cell lymphoma malignant cells are positive for CD20, CD45, and CD3.
Cytogenetics: many hematological malignancies have translocations in their genome that are diagnostic and very informative once detected. It is likely always a good idea to perform cytogenetic analysis on malignant cells in order to save time in making the diagnosis.
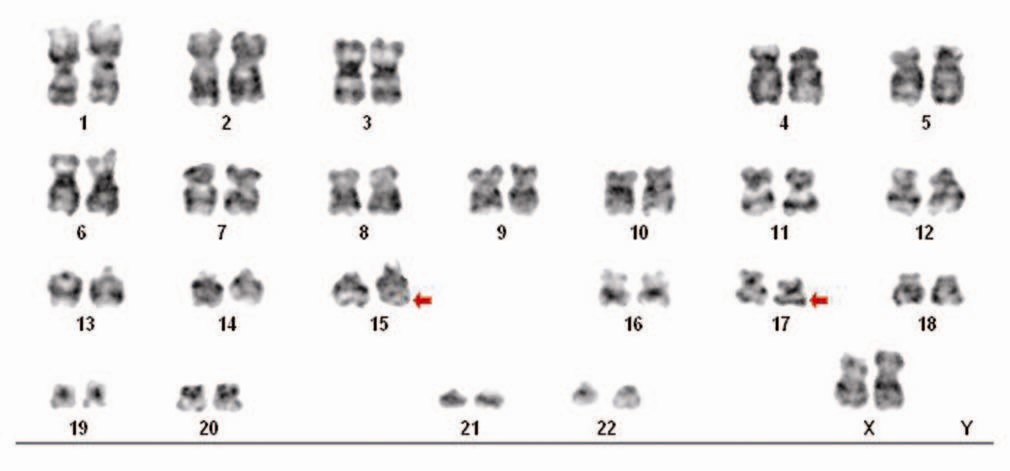
- t(3;14): diffuse large B-cell lymphoma IgH;BCL6
- t(8;14): is seen in Burkitt lymphoma (this activates c-myc)
- t(9;22): Philadelphia chromosome seen in CML (BCR-ABL fusion)
- t(11;14): Mantle cell lymphoma (cyclin D1 activation)
- t(14;18): Follicular lymphoma (BCL-2 activation)
- t(14;variable): multiple myeloma (IgH;MYC, cyclinD1, MAF, FGFR1)
- t(15;17): AML subtype acute promyelocytic leukemia
Page Updated: 01.16.2016