Page Contents
WHAT IS IT?
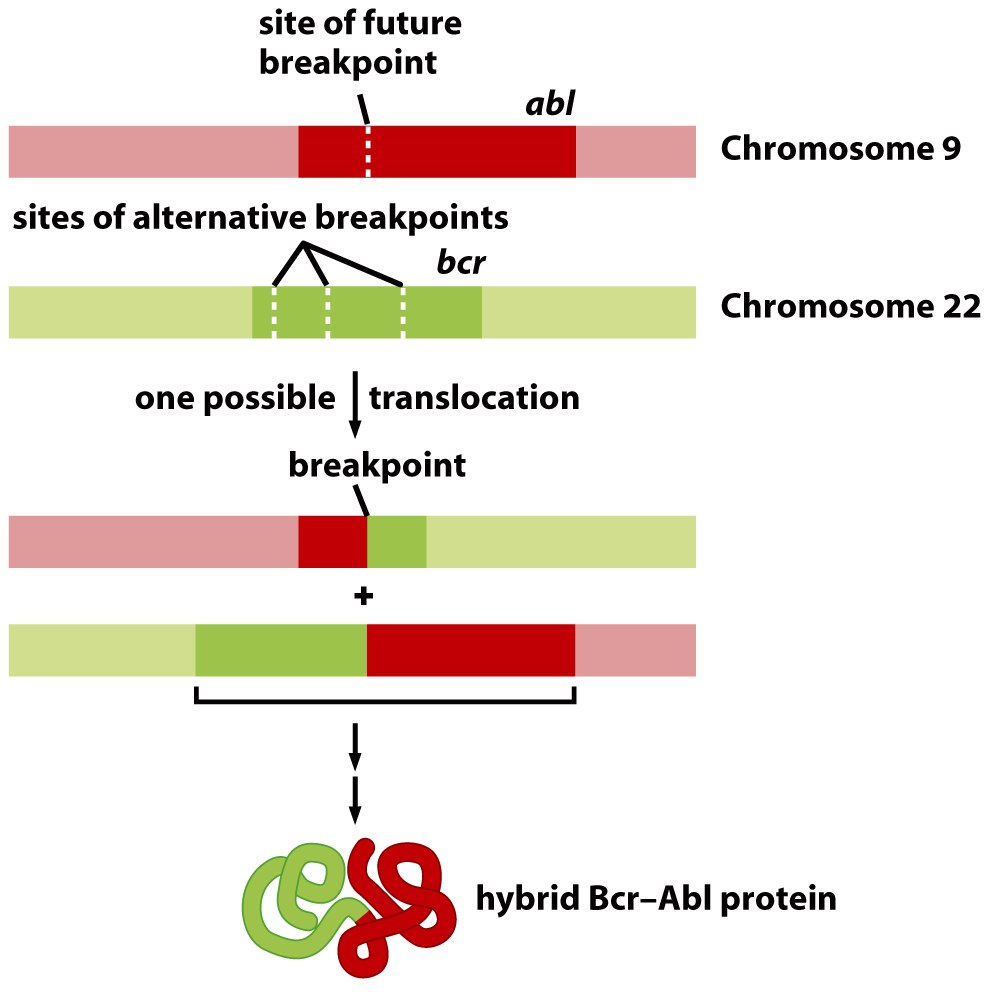
Chronic myeloid leukemia (CML): is a clonal hematopoietic stem cell cancer that is caused by the Philadelphia chromosome (a translocation between chromosomes 9 and 22). This is a disease of slower progression and insidious onset (as the name suggests) as opposed to acute myeloid leukemia (AML).
WHY IS IT A PROBLEM?
This rearrangement of chromosomes combines the genes ABL (chromosome 9) and BCR (chromosome 22) to create the BCR-ABL oncogene. ABL is a tyrosine kinase that becomes constitutively active after this fusion with BCR. Downstream of the tyrosine kinase pathway are many cellular mechanisms that promote growth, which ultimately become oncogenic in the setting of CML.
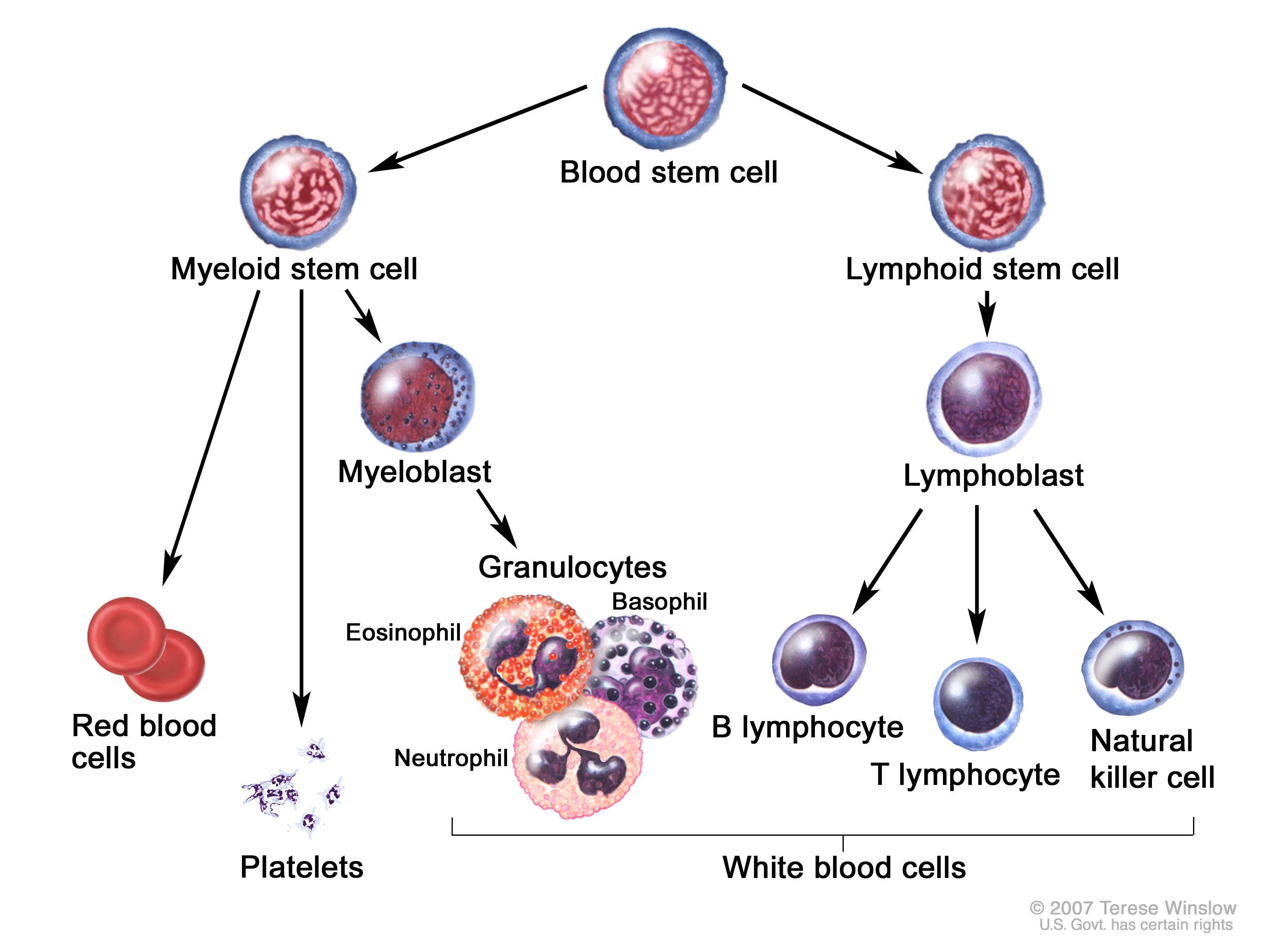
As the name implies, CML results in the overproduction of myeloid white blood cells/WBCs (most specifically granulocytes: neutrophils, basophils. eosinophils). As these cells become rapidly produced in the bone marrow there are a number of consequences that result in the clinical picture we observe when patients present with CML:
- Hypermetabolism: this rapid turnover in the bone marrow takes a significant amount of energy (can lead to fatigue).
- Anemia/thrombocytopenia: in some patients overproduction of the WBCs in the bone marrow can “crowd out” the production of red blood cells/platelets (anemia can further contribute to fatigue)
- Production of immature WBCs: given the rapid production of cells, many immature myeloid lineage precursors are released into the bloodstream that do not operate as fully functional granulocytes.
Phases of disease: chronic (when most patients present), accelerated, and blast phases that mark levels of disease progression (the more advanced the stage, the worse the symptoms above are)
WHAT MAKES US SUSPECT IT?
*Diagnosis is incidental in many cases, based on leukocytosis (increased white blood cell count) on complete blood count performed for unrelated reason
Risk factors: older adults
Chief complaints (chronic phase): below are some of the common presenting symptoms of patients with chronic CML
- night sweats
- fatigue
- malaise
- weight loss
- weakness
Chief complaints (accelerated/blast phases): below are some of the common presenting symptoms of patients with more advanced CML
- bone pain
- lymphadenopathy (swollen lymph nodes)
- skin infiltration
- extramedullary mass (chloroma/tumor composed of immature white blood cells)
Splenomegaly may be the only physical exam finding for many patients
HOW DO WE CONFIRM A DIAGNOSIS?
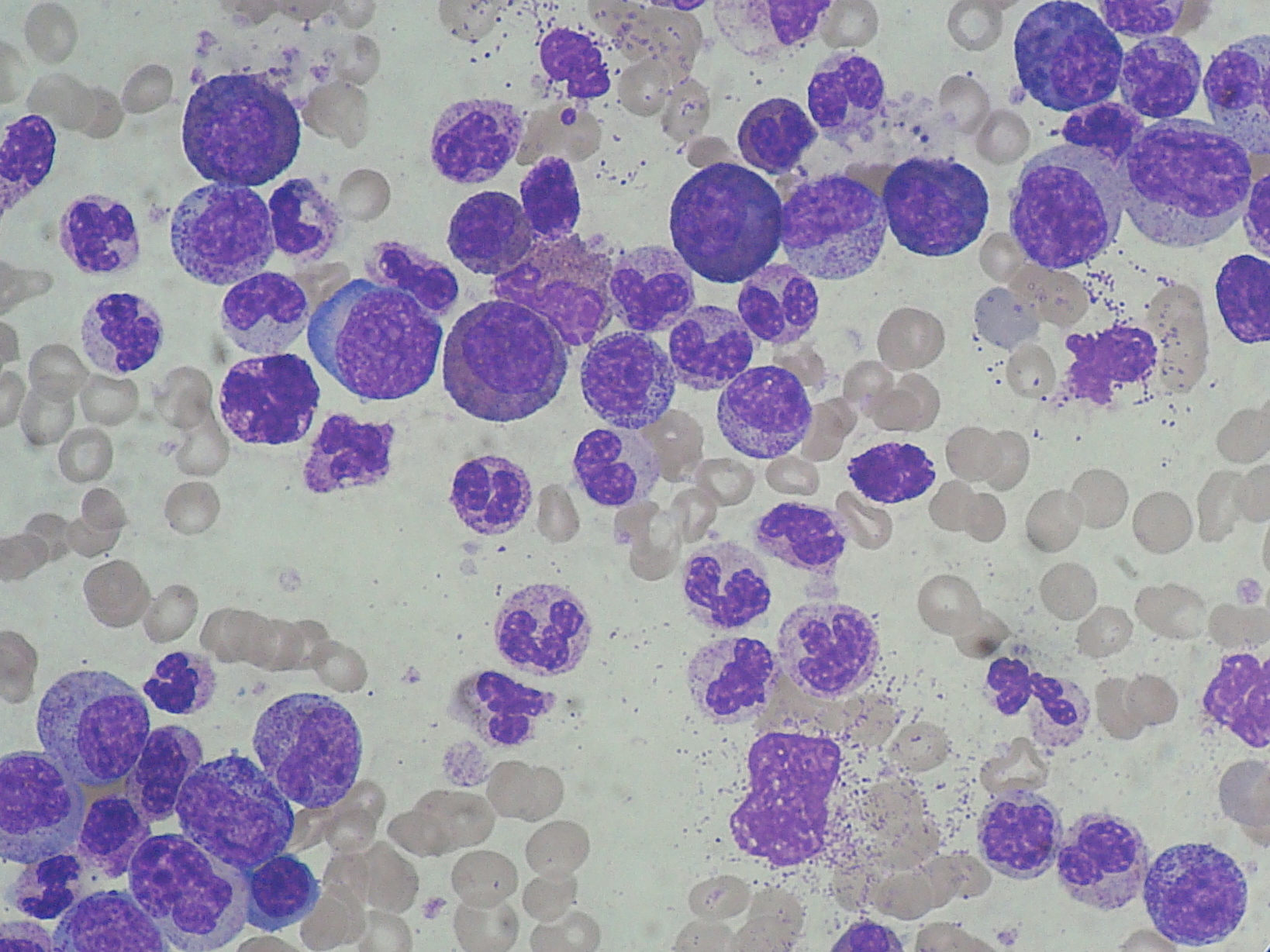
Complete blood count: increased neutrophils, basophils, and precursor cells myeloid cells (metamyelocytes)
- Anemia (decreased hematocrit/hemoglobin) and thrombocytopenia (decreased platelets) can be seen in some patients.
- Thrombocytosis (elevated platelet counts) can be seen in roughly half of patients (unique to CML, other leukemias will present with only thrombocytopenia).
- Increased WBC count: (~50,000 to 200,000 cells/mm^3). Specific increase of granulocytes can be observed.
Blood smear: will show a mix of neutrophils, metamyelocytes (neutrophil precursor), and increased levels of basophils and eosinophils.
Decreased LAP (Leukocyte Alkaline Phosphatase): enzyme found in white blood cells (WBCs) that is decreased in CML due to the production of so many immature WBCs.
Bone marrow biopsy: this can demonstrate not only the presence of CML, but an increase in myeloblasts (granulocyte precursor) to above 20% can demonstrate blast crisis. This is a phase of CML that represents an acute leukemia with very rapid division of myeloid cells. Normally fewer (less than 10%) of myeloblasts are present in CML during the chronic phase. Blast crisis of lymphoblasts in a similar way is also possible (representing ALL).
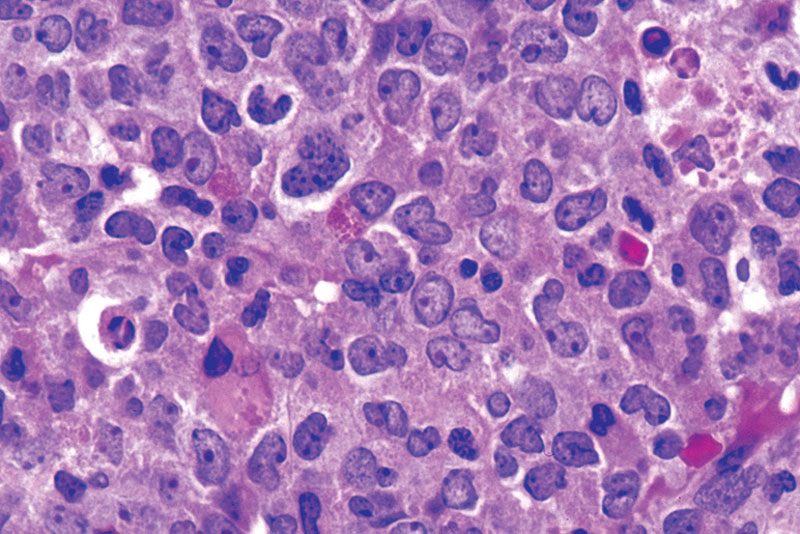
*No Auer rods will be present in blast cells (helping distinguish a CML blast crisis from AML).
Presence of Philadelphia chromosome is ultimately diagnostic for this condition (either by staining or PCR).
HOW DO WE TREAT IT?
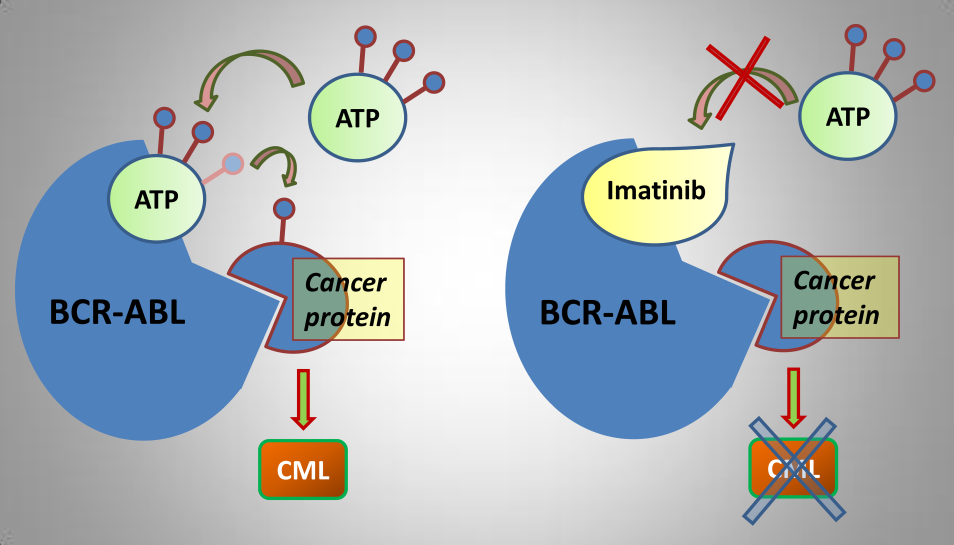
Imatinib (Gleevec): this is a tyrosine kinase inhibitor that targets the ABL kinase.
Allogenic bone marrow transplant: for patients who no longer respond to tyrosine kinase inhibitors
HOW WELL DO THE PATIENTS DO?
If left untreated CML has a slow progression (median survival of ~3 years without treatment). Imatinib works well and has a 5 year survival rate of ~90% for patients.
WAS THERE A WAY TO PREVENT IT?
There is no clear way to prevent this condition.
WHAT ELSE ARE WE WORRIED ABOUT?
CML can transform to either AML or ALL. Important to keep in mind that this disease can undergo blast crisis (which can be either myeloblasts or lymphoblasts)
OTHER HY FACTS?
Imatinib is the first generation of tyrosine kinase inhibitors. Newer inhibitors have been developed and more are still in development.
ARCHIVE OF STANDARDIZED EXAM QUESTIONS
This archive compiles standardized exam questions that relate to this topic.
Page Updated: 01.09.2016